Organic Qualitative Analysis
Organic qualitative analysis is the more complex of qualitative analysis. Typical organic molecular structures may incorporate aliphatic chains or rings, aromatic rings, ether linkages, heteroatoms, double and triple bonds, and alcohol, carbonyl, and carboxyl functional groups. Organic qualitative analysis usually identifies the presence of functional groups by a certain color change or reaction result, which allows us to classify compounds into homologous families. Instrument qualification methods are essential and typically include gas-liquid chromatography and electrophoresis, as well as infrared, ultraviolet and mass spectrometry. Qualitative analysis of organics requires comprehensive knowledge of a large number of chemical reaction types, as well as a general understanding of instrumentation and the ability to read spectra. Knowledge of chemical stability is essential as some tests can damage or destroy samples or present safety concerns.
Our Services
Test for Alcohols
General tests for alcohols include their reactivity with phosphorus chloride or sodium metal, in which they will effervesce. Another useful test is to put some alcohol into a concentrated sodium hydroxide solution or sodium carbonate alcohol solution. Our chemists are able to perform a series of reactions to monitor the color change to determine which type of alcohol is present, be it primary, secondary or tertiary.
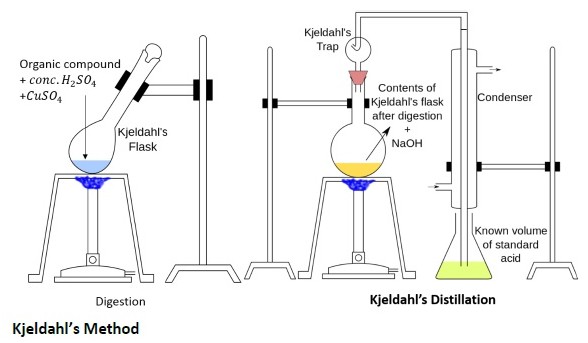 Figure 1. Estimation of Nitrogen by Kjeldahl Method.
Figure 1. Estimation of Nitrogen by Kjeldahl Method.
Test for Carboxylic Acids
Like alcohols, carboxylic acids effervesce when in contact with sodium metal or phosphorus chloride.
Test for Aldehydes and Ketones
There are two solutions commonly used to test for aldehydes. One is Tollen's reagent (ammonia silver nitrate solution). The other is Fehling's solution (alkaline copper solution). Aldehydes will always react with these solutions and form distinct products. These tests can also be used to distinguish aldehydes from ketones in a simple manner, since ketones do not react with these solutions. Therefore, no color change can be observed in any of the tests performed with ketones.
Test for Alkenes
An important test for testing alkenes is adding them to brominated water. If in the process of the reaction, the solution becomes lighter in color (from brown to colorless), the organic compound is an alkene.
General Scheme of Analysis
- Preliminary Tests
Note physical characteristics - solid, liquid, colour and odour
Perform an ignition test (heat small amount on metal spatula) to determine whether the compound is aliphatic or aromatic - Physical Constants
Determine the boiling point or melting point. Distillation is recommended in the case of liquids - Analysis for Elements Present
- Solubility Tests
 Figure 2. Summary of Qualitative Analysis of Organic Compounds
Figure 2. Summary of Qualitative Analysis of Organic Compounds
Instrumentation for Organic Analysis
- Gas Chromatography (GC)
Used for quantitative determinations of volatile and/or semi-volatile organic compounds.
- HPLC (High Performance Liquid Chromatography)
Used for quantitative or semi-quantitative determinations of organic compounds.
- Gel Permeation Chromatography (GPC)
Determines the fingerprint distribution of polymers by size and defines the average molecular weight of polymers.
- High Performance Thin Layer Chromatography (HPTLC)
A semi-quantitative technique that allows quick scan to check the purity of compounds. It is especially useful for dyes and/or materials that have UV absorption.
- Mass Spectromete (MS)
Integral molecular weight of a compound and the structure of unknown compounds can be deduced.
- NMR - Nuclear Magnetic Resonance Spectrometer
Used for qualitative and semi-quantitative analysis of organic compounds.
Used to analyze for a variety of NMR active nuclei (15N, 17O, 19F, 31P, 29Si, etc.). The most common nuclei analyzed are 1H, 13C and 31P.
- Infrared Spectrometer (IR)
Provides qualitative and semi-quantitative information about chemical bonds in the samples analyzed. An ATR accessory (with Ge/Si crystal) allows direct analyses of samples (paper, polymers, fibers,liquids, etc.) and their surfaces.
- UV-Visible Spectrophotometer (UV-Vis)
Used for qualitative or quantitative analysis of organic and inorganic materials.
- Extractor
Used to extract analytes from fabrics, fibers, polymers, soils, foods, etc.
- Freezer Mill
Used for analyze polymer pellets or films, fibers, etc.
References
- BYJU'S: IIT JEE Study Material
- STUDYLIB